Description
Vitamin C, also known as ascorbic acid, is much more than just an antioxidant to support our immune system as we’ve been taught.
It is primarily a redox balancer which means that it regulates the gain and loss of electrons of various molecules in various biological systems. For example, vitamin C is needed to make hydrogen peroxide by immune cells to kill pathogens. Vitamin C however, is also needed to neutralize any excess is made. Vitamin C is involved in renewing vitamin E levels.
We live in a microwaved world that we cannot escape, and this lowers our redox potential (Reduction-Oxidation) of our mitochondria. Quite literally, the mini-sun in our cells, , the mitochondria, requires a balanced redox state to generate energy and maintain the electrical effects of our bodies
Ascorbate is the negatively charged anion of ascorbic acid, and it is found in almost every tissue of the body. By taking ascorbic acid, we increase our plasma levels of ascorbate which then transfers into our tissues over the course of several months if supplementation is consistent.
Simply put, ascorbate helps replenish tissue redox status and primes us to be more resilient in our modern world.
For the past decade or more, ascorbic acid/vitamin C has been under attack while “whole food vitamin C” has been promoted in its place.
Not only does the “whole food C complex” have no legitimate scientific backing… as it ignores thousands of clinical studies proving the vast array of benefits ascorbic acid contains. One of the most famous chemists, Linus Pauling, who pioneered many of the chemical concepts still used today (such as electronegativity), focused heavily on supplementing more ascorbic acid.
Pauling had demonstrated that adequate ascorbate levels are necessary for good cardiovascular function, and collagen function. In other animals that still produce adequate amount of vitamin C (mind you, just as ascorbate and not the “whole food matrix”), we rarely see diseases of the cardiovascular system or collagenous diseases.
The Riordan clinic is another example of real time evidence of the use of high dose vitamin C for conditions like cancer, and viral diseases. There is mounting evidence of the role of vitamin C in properly establishing an immune response to combat these conditions.
SOME PROPERTIES OF VITAMIN C AS ASCORBATE
Ascorbic acid by itself increases glutathione levels in red blood cells (Johnston, et al 1993).
Ascorbic acid by itself protects the liver and lungs of cigarette smokers (Ueta, et al 2003).
Ascorbic acid is involved in the synthesis and metabolism of tyrosine, folic acid and tryptophan, hydroxylation of glycine, proline, lysine carnitine and catecholamine. It facilitates the conversion of cholesterol into bile acids and thus regulates blood cholesterol levels. It increases the absorption of iron in the gut by reducing ferric to ferrous state. As an antioxidant, it protects the body from various deleterious effects of free radicals, pollutants and toxins.
When combined with bioflavonoids, ascorbic acid has synergistic effects.
600 milligrams of ascorbic acid with bioflavonoids was administered three times a day to patients with recurrent herpes labialis and there was a remission of symptoms (Terezhalmy, et al 1978).
In patients with progressive pigmentary purpura (PPP), 500 mg of ascorbic acid with 50mg of the bioflavonoid rutin twice a day saw complete clearance of skin lesions (Reinhold, et al 1999).
Deficiency of vitamin C is often associated with anemia, infections, bleeding gums, scurvy, poor wound healing, capillary hemorrhage, muscle degeneration, atherosclerotic plaques and neurotic disturbances. For the correction of deficiency, vitamin C is often supplemented in large doses and unlike fat soluble vitamins, toxicity is rare (Chambial, et al 2013).
According to biochemist Irwin Stone, humankind as a whole has a genetic disease called hypoascorbemia. Considering we don’t actually synthesize our own vitamin C, this is very likely to be true.
“‘Full correction’ of this genetic disease is defined as supplying to the afflicted individual ascorbic acid in amounts the liver would be synthesizing had this mutation not occurred. It is assumed that the ascorbic acid requirements in man’s physiology are not much different from those of other mammals capable of carrying out this synthesis. Thus, the required amounts would fluctuate because ascorbic acid production in the mammals is regulated in accord with the superimposed stresses. Based on the scant data on mammalian synthesis, presently available only for the rat, a 70 kg individual would produce 1.8 gm to 4.0 gm of ascorbic acid per day in the unstressed condition. Under stress, up to 15.2 gm could be produced daily. When this estimated daily production of many grams per day is contrasted with the recommended adult daily intake of 70 mg, considered as adequate under the ‘vitamin’ hypothesis, the disparity is striking.”
It is an important point to note that most mammals produce their own ascorbic acid endogenously but humans do not. This is due to the lack of the enzyme L-gulonolactone oxidase in our liver that converts glucose to ascorbic acid.
When we combine toxic metal exposure, multiple chemical exposures, increasing EMF exposure, increasing overall stressors, and increasing demand on our physiology based on our environment, it is likely that 70 mg a day of ascorbic acid is not nearly enough.
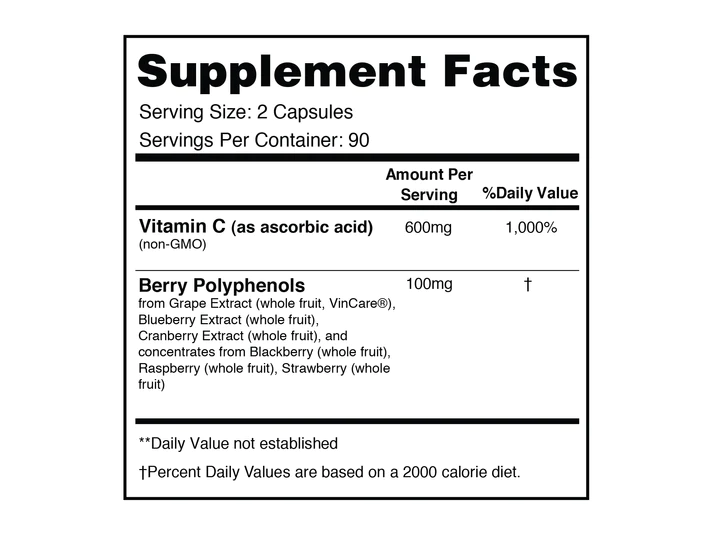
Mitolife Vitamin-C With Polyphenols provides 600mg of ascorbic acid and 100mg of bioflavonoids from multiple berry extracts per serving.
SUGGESTED USAGE:
Take 3 servings per day (6 capsules total)